Is Antibody Test For Covid 19 Fda Approved
In line with the ongoing response to the increasing number of COVID-19 cases in the Philippines the Food and Drug Administration FDA- Philippines hereby provides an initial list of approved Rapid Antibody Test Kits. Food and Drug Administration FDA is.
Fda Approved Antibody Test Could Test 2 000 New Yorkers Per Day
High-quality antibody tests a type of serological test.
Is antibody test for covid 19 fda approved. On May 19 2021 the FDA issued a safety communication reiterating that antibody testing should not be used to evaluate a persons level of immunity or protection from COVID-19 at any time and especially after the person received a COVID-19 vaccination4 Currently authorized SARS-CoV-2 antibody tests including the SARS-CoV-2 Semi-Quantitative Total Antibody assay 164090. Information about serological test performance characteristics are displayed in this Independent Evaluation of SARS-CoV-2 Antibody Test Performance. The Food and Drug Administration gave emergency approval to a COVID-19 antibody test that boasts near-perfect accuracy the company said Sunday.
At Home Covid 19 Tests Expert Opinions And. Are antibody tests used to diagnose COVID-19. 583 rows Coronavirus COVID-19 Update.
FDA APPROVES RAPID ANTIBODY TEST KITS FOR COVID-19. An antibody test does not detect the presence of the SARS-CoV-2 virus to diagnose COVID-19. Antibody drugs have been a standard treatment for Covid-19 infections for over a year but the AstraZeneca drug is the first intended for long-term prevention against COVID-19 infection rather.
In this image from undated video provided by AstraZeneca in December 2021 a worker packages the companys Evusheld medication. The Food and Drug Administration FDA approves the use of five 5 Rapid Test Kits for COVID-19 today March 30 2020. The very best prices available today fast delivery.
The US Food and Drug Administration FDA has granted emergency use authorisation to AstraZenecas monoclonal antibody treatment for COVID-19 among people with weakened immune systems. The FDA has authorized the use of some tests by certain laboratories under Emergency Use Authorization EUA. Fda Approved Covid 19 Antibody Tests List And Save Your money.
Antibody testing is not currently recommended to assess for immunity to COVID-19 following vaccination. FDA authorizes use of AstraZeneca COVID-19 antibody cocktail. Scientists are using these antibody tests to learn more about the level of antibodies needed to protect people from COVID-19 threshold of.
FDA Authorizes First Point-of-Care. Coronavirus Disease 2019 Testing Basics Fda. Covid 19 Tests And Collection Kits Authorized By The Fda In 2020 Infographic Fda.
Covid 19 Viral Testing And Fda Approved Antibody Testing In Bay County. To date such. Food and Drug Administration FDA has.
May 4 2020 Kim Schive. Have been granted FDA emergency use authorization EUA to detect antibodies to SARS-CoV-2. 26 rows COVID-19 ELISA IgG Antibody Test 04152020.
The combination of drugsbamlanivimab and etesevimabis authorized to treat mild-to-moderate COVID in children who are at high risk of becoming. On May 19 2021 the FDA issued a safety communication reiterating that antibody testing should not be used to evaluate a persons level of immunity or protection from COVID-19 at any time and especially after the person received a COVID-19 vaccination4 Currently authorized SARS-CoV-2 antibody tests including the SARS-CoV-2 Semi-Quantitative Total Antibody assay 164090. Have COVID-19 tests been approved by the Food and Drug Administration FDA.
The Food and Drug Administration FDA has extended its emergency use authorization EUA for the combination of two monoclonal antibody drugs to treat COVID-19 in all children including newborns. In a recent post we warned readers against rushing out to purchase one of the many COVID-19 antibody tests suddenly flooding the market noting that few of these tests have been independently validated and many are grossly inaccurate. Thats why today the FDA issued an update to a policy from March 16 2020 on antibody tests for COVID-19.
But what about the tests the US. Antibody Testing Is Not Currently Recommended to Assess Immunity After COVID-19 Vaccination. This test has not been FDA cleared or approved but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories.
AstraZenecas Evusheld an injectable monoclonal antibody cocktail has been approved for pre-exposure prophylaxis or PrEP against COVID-19 among. The FDA issued an emergency use authorization Wednesday for AstraZenecas antibody cocktail Evusheld for what is known as pre-exposure prophylaxis or PrEP against Covid-19. The antibody tests and the molecular tests together referred to as tests have not been cleared or approved by the Food and Drug Administration FDA.
This test has been authorized only for detecting the presence of IgG antibodies against SARS-CoV. 17 hours agoOther monoclonal antibodies granted emergency use authorization EUA by the FDA are delivered by intravenous infusion to people already infected by the COVID-19 virus to prevent severe illness or. The FDA supports all efforts to address this pandemic.
Was That Covid 19 Antibody Tests Promise Answers But Beware Of Their Limits Commonhealth. FDA approves new COVID-19 antibody drug to protect most vulnerable from coronavirus By Agencies Vaccine makers race to update COVID shots. Tests with FDA Emergency Use Authorization have varying degrees of accuracy.

Fda Advisory No 2020 483 Fda Approves Rapid Antibody Test Kits For Covid 19 Food And Drug Administration

Fda Approves Rapid Antibody Test Kits For Covid 19 Food And Drug Administration
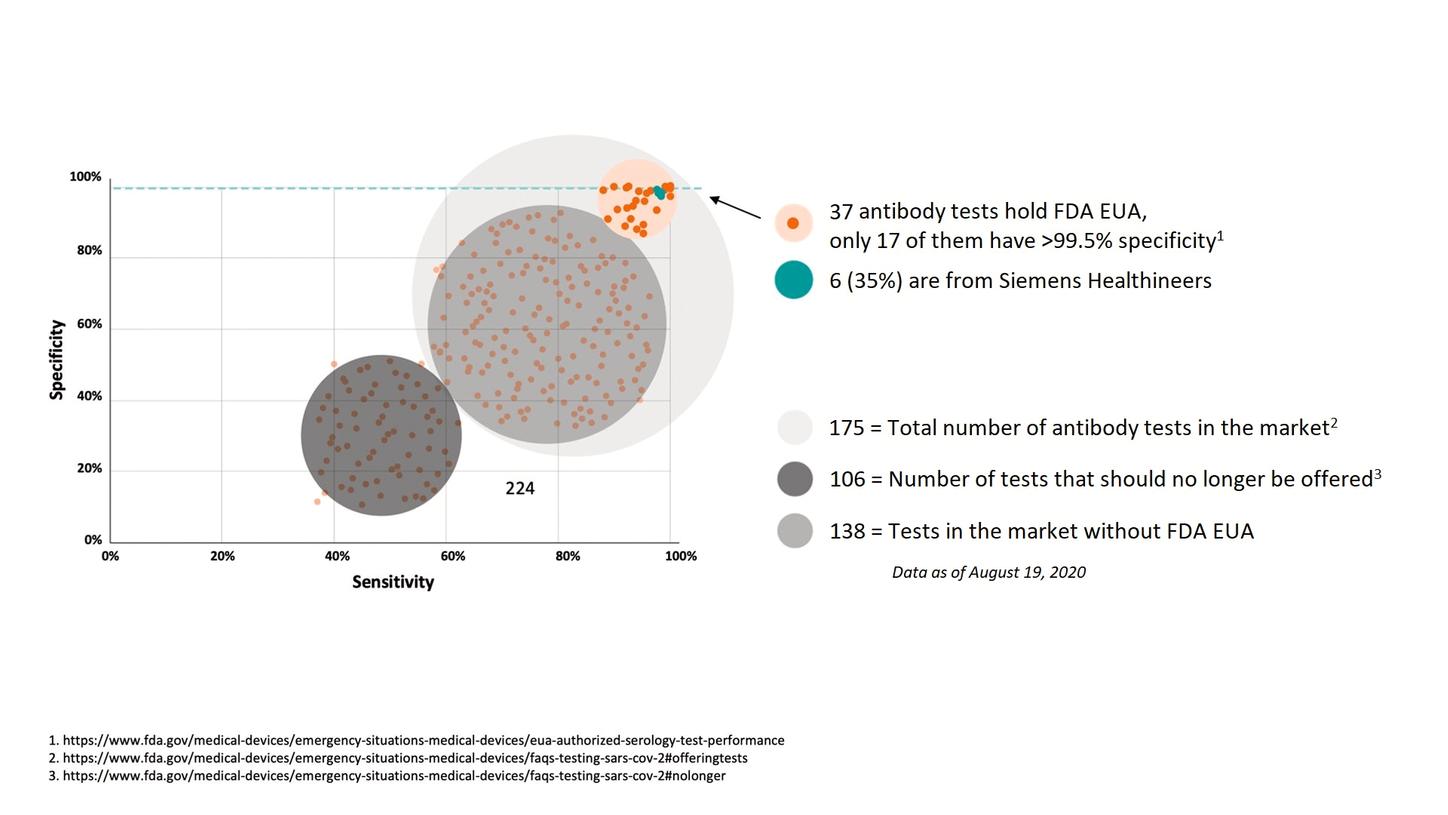
Antibody Testing Is Critical In Overcoming The Covid 19 Pandemic Now And In The Future

How Accurate Are Fda Approved Covid 19 Antibody Tests Mit Medical
Posting Komentar untuk "Is Antibody Test For Covid 19 Fda Approved"